Vascular dysfunction in hyperhomocysteinemia
This project originated from studies in this laboratory on prothrombotic effects of elevated levels of homocysteine, an amino acid that is associated with coronary artery disease, stroke, peripheral vascular disease, and venous thrombosis. We were among the first to demonstrate impaired vascular function in moderate hyperhomocysteinemia. Current studies are directed toward investigating the mechanisms of vascular dysfunction in hyperhomocysteinemia and atherosclerosis using molecular genetic approaches. Supported by the Department of Veterans Affairs, the National Institutes of Health, and the American Heart Association.
Homocysteine Metabolism
Oxidative Stress in Hyperhomocysteinemia
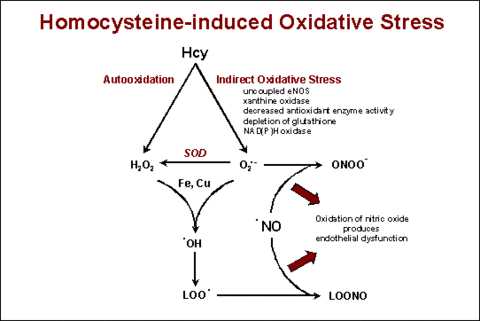
Autooxidation of homocysteine may produce hydrogen peroxide (H202). Indirect oxidative effects of hyperhomocysteinemia may include generation of superoxide from uncoupled endothelial nitric oxide synthase (eNOS), xanthine oxidase, or NAD(P)H oxidase, downregulation of antioxidant enzymes, and depletion of intracellular glutathione. In the presence of transition metals such as copper (Cu) or iron (Fe), H202 and superoxide (O2·-) may react to form hydroxyl radical (·OH). Hydroxyl radical may promote the generation of lipid peroxyl radicals (LOO·), and both superoxide and lipid peroxyl radicals may react rapidly with endothelium-derived nitric oxide (·NO) to produce peroxynitrite (ONOO-) or lipid peroxynitrites (LOONO), respectively. SOD, superoxide dismutase; ONO-, nitrite.
Structure and function of thrombomodulin
This project originated from our discovery that the anticoagulant protein thrombomodulin is expressed in a highly regulated fashion by keratinocytes in squamous epithelium. Our recent studies suggest that thrombomodulin regulates tissue repair during cutaneous wound healing. Current studies are directed toward investigating the function of thrombomodulin in endothelium and epithelium using gene targeting approaches. Supported by the Department of Veterans Affairs.
Expression of Murine Thrombomodulin in Keratinocytes During Cutaneous Wound Healing
Platelet signal transduction by thrombin and collagen
Thrombin is a multifunctional serine protease that acts as a pivotal enzyme in blood coagulation. We have characterized thrombin receptor signal transduction in platelets and T cell lines, and demonstrated counter-regulation between thrombin receptors and the T cell antigen receptor. These observations support the model that thrombin provides a functional link between hemostasis and immune activation. We have demonstrated that the adapter protein SLP-76 ("SH2 domain containing leukocyte phosphoprotein of 76 kD") is essential for platelet signal transduction in response to collagen, but not thrombin. Currently, we are using genetic approaches in mice to define the hemostatic consequences of impaired collagen signal transduction. Supported by the American Heart Association, the Howard Hughes Medical Institute, and the National Institutes of Health.
Activation of Murine Platelets by Collagen and Thrombin
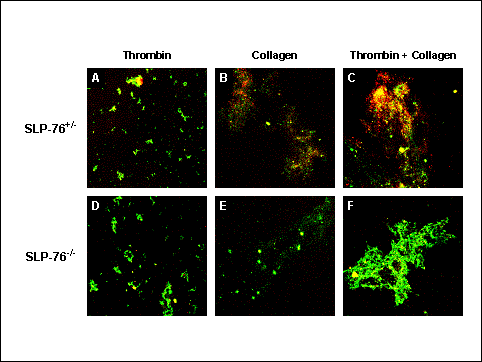
Confocal photomicrographs of annexin V binding with fibrinogen-adherent platelets. Following adherence to fibrinogen-coated coverslips, SLP-76+/- (A, B, C) or SLP-76-/- (D, E, F) platelets were stimulated with thrombin (0.5 U/ml) (A, D), collagen (50 mg/ml) (B, E), or thrombin plus collagen (C, F). Adherent platelets and platelet aggregates were identified by staining with FITC-anti-CD49b (green fluorescence) and annexin V binding was detected by staining with PE-conjugated annexin V (red fluorescence). Yellow fluorescence indicates co-staining for both CD49b and annexin V binding.